ABOUT ENTEGRIS
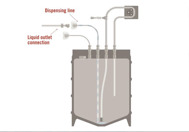
In biopharmaceutical production, each instance where materials or products in development must be stored, handled, or transported presents a risk for breakage, contamination, and expensive product loss. In a modern, decentralized biopharmaceutical production line, materials and products must be kept frozen to ensure product safety, consistency, and shelf life. Because of these new realities, we face more production costs, logistics, speed, and complexity.
Bioproduction today has dramatically increased the value of product in each container. Production processes have been contracted out or may be taking place in different parts of the country or globe. At colder temperatures, containers have considerable limitations; traditional storage bottles are often more brittle at cold temperatures and can break easily, do not pack into containers or store in freezers efficiently and take longer to freeze and thaw due to their shape and volume.
Entegris is in a unique position to help overcome these cold temperature challenges by offering assemblies designed for extreme cold and by partnering with qualified equipment manufacturers specializing in reliable, cold-chain containment. Let us help you gain control over your processes and reduce the potentially enormous financial risk to your valuable products.
VIDEOS
-
Listen in to real-time questions and answers from the audience. During “Building An In-House Cell & Gene Manufacturing Facility – Best Practices & Lessons Learned.”
-
Take a look into how automated instruments promote process and product standardization, assist in eliminating the variable human factor of manual processes, and address the complexity of manufacturing life-saving therapeutics.
-
Does or should speed to clinic impact a company’s decision to build versus buy? Explore this question and others along with lessons regarding capacity and meeting anticipated demand.
-
When building a production line from lab-based processes, sometimes staff primarily familiar with research might not know the specifics of regulations relating to late-stage drug production.
-
Learn about some of the major milestones and timelines that must be met throughout the building process with a pharmaceutical manufacturing expert.
-
Manufacturers know that an empty GMP facility costs a great deal of money, so how can companies balance the cost and labor model.
-
Are you and your manufacturing team ready to take on the demands of Good Manufacturing Practices, compliance, and regulatory approval?
-
CMC experts offer some forward-looking thoughts on how AI and Machine Learning will impact CMC, and CMC-related regulatory interactions, in the not-too-distant future.
-
CMC experts discuss what forces and organizations are defining CMC requirements for biologic therapies moving forward.
-
How important is early CMC work in the grand scheme of what will ultimately become a submitted CMC package?
-
CMC experts discuss the advantages of data-driven CMC packages in regulatory preparation and during regulatory interactions.
-
CMC experts answer audience questions about the cost of advanced PD/CMC applications in biopharma manufacturing, and share perspectives on how to test and validate the new technologies in today’s regulatory environment.
-
Experts address freeze/thaw considerations and discuss who’s driving the adoption of data-driven PD and CMC applications.
-
In this segment CMC experts address the tools they, and their peers, are leveraging to achieve CMC data efficiency.
-
An effective CMC-process development infrastructure is critical to biopharma success, because what good is a great PD model if it won’t scale?
-
Experts kick off the Bioprocess Online Live event Data-Driven CMC For PD Speed, Regulatory Efficiency in this segment covering when and where new biopharmas should start building out a CMC strategy in earnest.
-
Watch how our single-use mixing system creates a completely sterile mixing and recirculation process, ensuring your buffer and media are mixed correctly and efficiently.
-
Ross Acucena, applications director, and Donnie Beers, product manager, introduce our single-use mixing system made to be deployed rapidly to help manufacturers meet development, supply, and cost objectives.
-
Ross Acucena, applications director, and Donnie Beers, product manager, introduce our highly functional and adaptable microcarrier cell separation bags designed for single-use processes.
-
This video demonstrates the Aramus™ 2D single-use bag assemblies reliability in a frozen drop test comparing the fluoropolymer bag to a polyethylene bag.
-
This video shows examples of the performance of Aramus™ film, polyethylene, and polyolefin film when subjected to cold temperatures.
-
Janelle Rupkalvis from Entegris Life Sciences describes the options, technologies and suppliers for single use assemblies in low temp bioprocessing.
-
Janelle Rupkalvis describes the process and options for ordering single use assemblies from Entegris Life Science.
DATASHEETS
-
This solution reduces the risk of contamination by aseptically handling samples or aliquots of liquid that would normally have to be collected via open sampling methods such as conical tubes or vials.
-
Improve your handling, efficiency, throughput, and total cost of ownership in bulk-drug substance and cold-chain applications through a reusable, plastic shell solution.
-
This cell banking manifold kit can shorten your seed train process from weeks to days with manifolds that enable biomanufacturers to save time, money, and space while reducing contamination risks during upstream processing.
-
Receive the fluid handling benefits of customization, fast design, and delivery with quality assembly solutions.
-
Choose the correct bioprocess powder bag to serve as your operations containment and transfer solution, by reviewing the features, benefits, and specifications of this single-use option.
-
Learn how this buffer capsule filter provides end users with the flexibility to scale their templates up and out, improve process economics, enable multiuse facilities, and reduce contamination risks.
-
Aramus™ 2D single-use bags that provide high purity, exceptional compatibility, and increased safety for critical process fluids and final products are now available in custom shapes, sizes, and assembly configurations.
-
Our technology center has everything needed to make educated decisions about what freezing process and products best fit your needs.
-
PSTP series cartridge filters ensure reliable product processing featuring polypropylene components and a hydrophilic polyethersulphone (PES) membrane enabling economical and efficient use in various pharmaceutical applications.
-
PSTP series cartridge filters ensure reliable product processing enabling economical and efficient removal of particulate and colloidal contaminants in various pharmaceutical applications.
-
Made from high-quality stainless steel and heavy-duty plastic material, the freezing shells provide greater bulk drug substance (BDS) protection during handling, more consistent freezing/thawing, and reduced storage density.
-
Entegris solutions allow you to protect and store your high-value process solutions with reduced risk of product loss, contamination, and compromised quality. Expanding our portfolio further, we offer LN2 (liquid nitrogen) cassettes for use with Aramus™ single-use bag assemblies.
-
The utilization of single-use (SU) systems continues to grow, and so do the purity concerns of the SU components and their potential impact on high-value final products. From the high cost of product loss due to assembly failures at fill/finish, to the ever-increasing scrutiny from regulatory bodies, every angle requires protective measures. Now, Entegris brings a new level of assurance to your process. The Aramus™ single-use 2D bags are made of a high-grade, gamma-stable fluoropolymer, providing higher purity, greater compatibility, and increased safety for critical process fluids and final products.
-
Two of the most significant benefits to single-use bioprocessing over reusable manufacturing equipment are lower cost and simpler operation. And yet some processing steps, such as mixing, have not been able to realize these benefits. The Entegris mixing system is designed to maximize simplicity, ease-of-use, and affordability.
-
Learn about a microcarrier separation system designed to ease the complexity and financial burdens of single-use systems while facilitating a separation process using a streamlined, single-use filter/mesh bag system and peristaltic pump.
-
These motion bioreactor bags bring the benefits of ultimate customization, fast design, and delivery to cell culture applications to your lab.
-
What does “custom” mean for bioprocessing companies? Many system suppliers advertise their capability in customization but in reality, limit it to the interchange of components or lengths of tubing. This is helpful, but falls short of what many end users are looking for. With single-use systems, Entegris offers true customization to meet your exact process needs, fast.
CONTACT INFORMATION
Entegris, Inc.
129 Concord Road
Billerica, MA 01821
UNITED STATES
Phone: 978 436 6500
Fax: 978 436 6735
Contact: Barry Westfall
FEATURED ARTICLES
-
Cell banking stands at the forefront of medical advancement. This guide delves into the steps involved in protecting these priceless resources, from cell collection and processing to storage and cell expansion.
-
An alternative to traditional polyethylene bags is offering cell and gene therapy manufacturers a solution to contamination concerns regarding DNA or RNA fragments.
-
Examine the benefit of using fluoropolymer film bags for the handling and storage of high value oligonucleotides- based payloads.
-
Entegris announces their collaboration with Agilitech, allowing them to offer an extended line of custom, single-use systems for cell and gene therapies.
-
Explore traditional cold-wall and convection technology to determine which best suits your cold needs for upcoming projects.
-
Compare single-use system bags and learn why both particulate levels and minimizing contamination are important for single-use systems.
-
Learn about the three most common sources of filtration failures and how to prevent these problems.
-
Learn about large and small volume parenteral injectable drugs, their comparative sizes, types, sterilization processes, and applications.
-
The FDA holds strict requirements for magnetic bead removal prior to therapeutic use. Learn how this system effectively separates cells from microcarriers and provides maximum cell recovery.
-
Examine one company's solution to minimizing production delays despite the single-use supply constraints exacerbated by the COVID-19 pandemic that are interfering with bioprocess operations.
-
Freezing biologics is a complex undertaking. As more biotherapies enter the market, ensuring manufacturers have the technology to optimize their cold chain applications is paramount.
-
The need for low-temperature storage capable of surmounting the molecular and logistical challenges associated with these treatments has become core to achieving commercial-scale production.
-
We investigate material adsorption of mRNA-encapsulated lipid nanoparticle (LNP) formulation comparing the Aramus fluoropolymer bag to commonly used glass vials and polypropylene (PP) cryotubes.
-
This application note explains the use a liquid particle counter to qualify the cleanliness of single-use 2D bag assemblies.
-
New single-layer assemblies, made without the curing agents, antioxidants, plasticizers, or adhesives that represent potential contamination sources, may offer companies a safer, more efficient choice.
-
This application note reviews the chemical compatibility of Aramus™ assemblies with dimethyl sulfoxide (DMSO), which is commonly used as a cryopreservant in the cell and gene therapy (CGT) markets.
-
This application note explores the cryogenic temperature compatibility of the Aramus™ bag assembly material through freezing and drop testing after 24-hour minimum immersion in the liquid nitrogen (LN2) vapor phase.
-
This application note demonstrates how the Nicomp Z3000 system is ideally suited for determining size and zeta potential of gold nanoparticles.
-
Understanding the cold chain supply scale-up challenges that can threaten product integrity empowers manufacturers to implement efficient, cost-effective solutions.
-
The size and surface charge of liposomes—spherical engineered particles made of phospholipids—are important characteristics that require measurement and monitoring in biopharmaceutical applications.
WEBINARS
-
Review data and evidence that demonstrates the benefits of an integrated approach to achieving critical end-user goals such as scalability, efficiency, sustainability, and optimized process economics.
-
Discover how to integrate appropriate materials and tools for oligonucleotide handling, optimize DNA storage using dedicated fluoropolymer film, perform complex biochemical reactions in bags, and more.
-
Explore how filtration manufacturers optimize materials for performance and integration within single-use systems, including the value of optimizing sterile filtration by design and how it integrates and maximizes performance.
-
Learn how utilizing high-density cell banking workflows in combination with your already intensified upstream process can further enhance your process productivity while reducing contamination risks.
-
Learn about particle sizing methodology, equipment, software, and compliance to meet USP standards for parenteral drug manufacture, proteins, ophthalmic solutions and lipid emulsions.
-
Entegris' Joy Xiaohui Chen and Cari Sadowski of Fujifilm Diosynth Biotechnologies evaluate single-use bag material interaction and adsorptive relationship with AAV viral vector during storage.
-
Learn more about the uncertainties and complexities encountered when expanding the application of single-use technologies (SUT) in cryogenic bioprocesses.
-
Study data shows the utility of low temperature suitable materials and packaging, sensors for monitoring shipments, and qualification and evaluation through simulated standards and real-world transit.
-
How high-density cell banking accelerates upstream processes by providing a larger volume of concentrated cells at the start of a production run, shortening seed training from weeks to days.
-
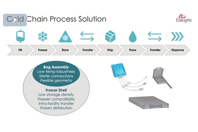
Explore the needs and requirements of equipment and processes to handle lower temperatures, incuding a complete, cold-resistant solution of bags, tubing, connectors, and shells for freeze/ship/thaw processes.
-
Mike Johnson from Entegris and Johannes Kirchmair from Single Use Support explain the importance of having a complete, cold-resistant solution of bags, tubing, connectors, and shells.