Designing Highly Agile Bio/Pharma Manufacturing Facilities
By Mark F. Witcher, Ph.D., biopharma operations subject matter expert

For many compelling reasons that include rapidly advancing therapeutic technologies, expedited regulatory reviews, adaptive clinical trials, pandemics, emergency use authorizations (EUAs), increasing alternative cell line options, and other 21st century technologies such as continuous manufacturing, biopharma manufacturing is frequently in the critical path for making important therapies available to patients.1,2 We have reached the point where a new manufacturing facility paradigm is required to support the rapid development and launch of many important new therapies.
This article describes how a facility designed as a matrix of nearly identical connected cleanroom elements can be operated to provide a highly agile manufacturing capability. Agile manufacturing is not achieved by modular facility designs. While modular facilities are an important method for rapidly and efficiently creating manufacturing capacity, the approach does not significantly improve a facility’s ability to rapidly manufacture a variety of products using diverse processes. Agile manufacturing is achieved through facility designs that support a wide variety of unit operations manipulated and operated as independent yet connected modules. Only a highly agile facility can efficiently support the simultaneous manufacturing of a diverse portfolio of products and processes.
Future manufacturing facilities must be truly multipurpose, capable of simultaneously supporting multiple products made by different processes at a wide variety of scales necessary to support a product’s entire development life cycle as it progresses through its process development, clinical trial, and launch phases to efficiently reach the patients. To be effective, transitions between products and processes should be measured in days, not months.
For a facility to support highly agile operations, it must:
- have operating areas that are agnostic to the process types and scales,
- be able to expand or reduce the number of areas required to implement any process and support functions to make any product at any scale,
- provide for separation or isolation of unit operations when required or needed,
- facilitate the efficient movement of personnel and materials between unit operations,
- facilitate the efficient addition and removal of process equipment to maintain a high overall facility utilization rate,
- support the rapid introduction and removal of unit operations without interfering with other ongoing products and processes,
- provide numerous options for transferring raw and in-process materials between unit operations, and
- rapidly isolate process failures from other products and processes.
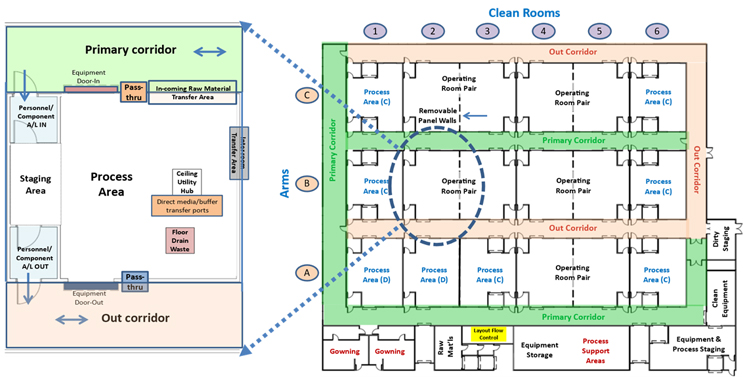
Figure 1 shows the basic layout strategy of a multipurpose facility (MPF). The MPF’s core shown on the right is composed of a matrix of essentially equivalent operating areas or cleanrooms that can be configured individually or in groups to support a wide variety of unit operations. On the left is one of the operating core’s elements showing the layout and features available in each area.
Figure 1: The MPF layout on the right has 18 elements arranged in three arms with six cleanroom elements per arm. Element locations are designated by arm and number. A single cleanroom element B2 is shown on the left. Essentially any skid-mounted process unit operation, including media and buffer preparation, can be operated in any area. Click on image to enlarge.
Every operating area is connected to a grade D primary corridor shown in green by an airlock and a rollup door that supports controlled bidirectional access to each room for normal operation and loading clean process equipment. Virtual ballroom operation can be achieved by leaving the rollup door between the primary corridor and operating areas open.
Each area is also connected to a CNC (controlled non-classified) out corridor shown in light orange by an airlock and rollup door for removal of waste materials and no longer needed process equipment when isolated from the primary corridor. The out corridor also provides the ability for staff or regulatory personnel to observe any area without accessing the clean classified areas of the facility. The layout would also support unidirectional flow in special situations.
Each element within the core is approximately 100 square meters, with some adjacent rooms having removeable dividing cleanroom panels that can be used to create larger operating spaces (operating pairs) when needed. Each area has support features located in the ceiling that contain an HVAC unit, utility panels, and access to interarea tubing connections to other areas if needed.
The operating core is located on a single level to facilitate construction, ease of operation, and simple maintenance access and to allow for topside access via a walkable mezzanine for adding interarea in-process and utility connections. Because of the MPF’s many connectivity options, many of a manufacturing process’s unit operations do not have to be adjacent to each other.
Connectivity between the operating areas can be achieved by:
- single use (SU) tubing to an adjacent area via wormholes or passthroughs,
- portable totes via the primary corridor, and
- simple stainless steel tubing pairs that run through the walkable mezzanine and are cleaned and sanitized by portable clean-in-place systems.
The primary limitation of the MPF operating paradigm is that all the equipment must be movable by being skid-mounted. With the aid of tugs, processes up to 2,000 liters can be easily mounted on one or more skids and transported through hallways to and from operating areas.
While the facility is designed to provide a wide variety of process connectivity options, the MPF provides unique options for supporting the flexibility of a ballroom while allowing either the segregation of an operating element or the immediate isolation of areas if necessary to prevent contamination of other products or processes, facilitate equipment removal, or perform maintenance of the facility or equipment.
Achieving Compliant Agile Operation
The design features of the operating core allow for a wide variety of quality management system (QMS) controlled area segregation options for introducing, operating, and removing processes that have been subdivided into logical operating units (LOUs). All manufacturing processes can be easily divided into groups of unit operations for distribution within the facility in a wide variety of configurations.
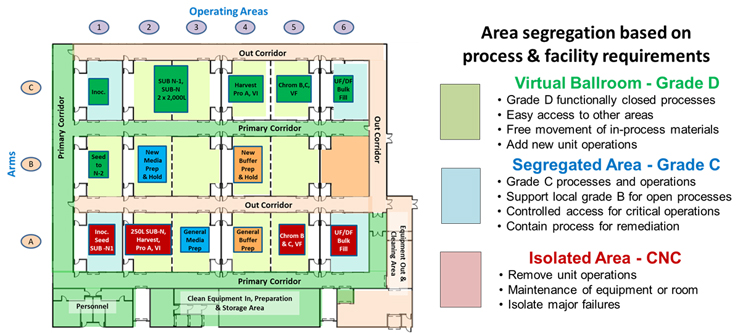
Based on a process’s needs, any cleanroom element in the core can be operated in one of three classification modes:
- Virtual Ballroom (grade D) – If the primary corridor rollup door is opened, the room can be operated as a virtual ballroom, allowing free access to the primary corridor.
- Segregated Cleanroom (grade C) – If the primary corridor rollup door is closed and the room is accessed via the airlock between the room and the primary corridor, then the room can be operated as a separated grade D space or as a grade C area to provide additional environmental segregation and greater physical control of the unit operations. The areas can achieve additional segregation by including local grade B environments using laminar flow hoods or isolators.
- Isolated Area (CNC) – If access to the primary corridor is closed and secured, and access is limited to and from the out corridor, the area is isolated from the operating core.
Although any area can be operated in any mode, Figure 2 shows an MPF in one particular configuration based on the needs of the current processes being operated within the facility. When any of the process unit operations are complete, the area can be switched to isolation mode and the equipment removed through the out corridor. After the area is cleared, it can be placed in segregated mode and cleaned. After cleaning, it can be placed in ballroom mode and loaded with the new process equipment to support the next product.
Figure 2: An 18-element MPF with light green areas shown in a ballroom configuration, except for elements A1, A6, C1, and C6, which are shown as segregated areas, and area B6, shown as isolated for maintenance or equipment clearance. Click on image to enlarge.
The ability to put any area in any of the three segregation modes provides the capability to manage a wide variety of operating risks. Areas with closed processes can be conveniently operated in virtual ballroom mode while processes that should be segregated can be readily operated in grade C spaces. Should any process suffer an operational failure, it can be immediately isolated for resolution of the problem via controlled access through the out corridor.
Since the MPF’s operating core is agnostic to the process or scale, it can be constructed with very little process information. MPFs can be designed and constructed in a wide variety of locations using modular construction methods in large existing buildings or on greenfield sites.
Flexible Design Options
MPFs can be constructed with any number of arms and elements per arm. Figure 3 shows a small single-arm facility with six elements. The six elements can be run in a wide variety of configurations that include upstream, downstream, laboratory, storage, or any other function needed by the company. The facility can be adapted to protein, vaccine, or nucleotide production based on your company’s product requirements. The MPF operating paradigm may also be applicable to other pharma manufacturing processes depending on their scale and equipment requirements. Autologous and allogenic cell processes, especially those using small volume movable isolator filling equipment necessary for rapidly freezing the cells, can benefit from the flexibility of the MPF.
Figure 3: An expandable six-element MPF for an emerging biotech company. Click on image to enlarge.
One feature of the MPF is that facilities with an odd number of arms can be expanded on the topmost out corridor if the site is properly planned. With predesigned QMS procedures, interruptions to the operating core during construction can be minimized.
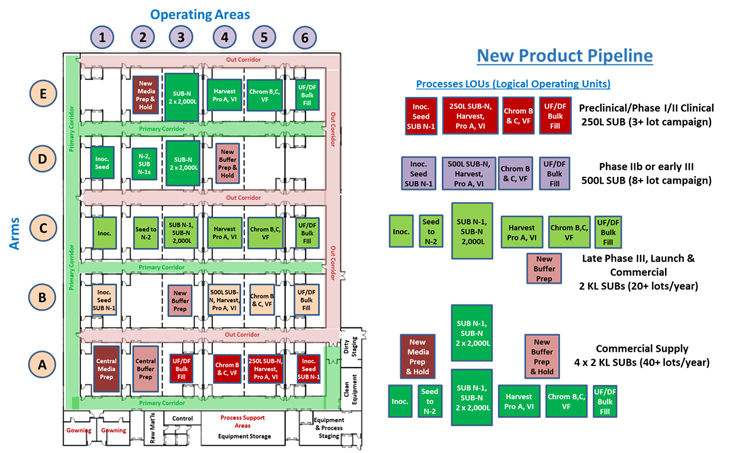
The full potential of an MPF as a dynamic, flexible manufacturing platform is shown in Figure 4. The facility is shown operating four different mAb processes with scales ranging from a 250 L SUB for early clinical production to a 4 x 2,000 L SUB commercial-scale process capable of producing very large quantities of product for building prelaunch inventory.
Figure 4: A very large 30-element MPF is shown on the left, with an example portfolio of processes shown on the right. Note that some of the process implementations require additional media and buffer capacity. Click on image to enlarge.
The large 30-element MPF would be ideal for a CDMO or major biotech company with a large pipeline. Such a facility would be capable of manufacturing many different products but also provide larger-scale manufacturing options to support manufacturing commitments necessary for receiving expedited review status of important new therapeutics.
The MPF operating paradigm provides a highly agile manufacturing capability for meeting the many challenges of manufacturing 21st century products that supply small to midsize niche markets, are at high risk of clinical failures, and have uncertain development timelines based on high product development uncertainties.
References
- Witcher, M. F. “How FDA’s 21st Century Goals can be realized by using a Multi-purpose Manufacturing Facility,” Pharmaceutical Technology, 2018. http://www.pharmtech.com/how-fda-s-21st-century-goals-can-be-realized-using-multi-purpose-manufacturing-facility-0?pageID=2
- Witcher, M. F. “The Facility Challenges of Developing Continuous Process based Biopharmaceutical Products,”, Pharmaceutical Engineering – iSpeak Blog, March 2019. https://ispe.org/pharmaceutical-engineering/ispeak/facility-challenges-developing-continuous-process-based-biopharma-products
About The Author:
 Mark F. Witcher, Ph.D., has over 35 years of experience in biopharmaceuticals. He currently consults with a few select companies. Previously, he worked for several engineering companies on feasibility and conceptual design studies for advanced biopharmaceutical manufacturing facilities. Witcher was an independent consultant in the biopharmaceutical industry for 15 years on operational issues related to: product and process development, strategic business development, clinical and commercial manufacturing, tech transfer, and facility design. He also taught courses on process validation for ISPE. He was previously the SVP of manufacturing operations for Covance Biotechnology Services, where he was responsible for the design, construction, start-up, and operation of their $50-million contract manufacturing facility. Prior to joining Covance, Witcher was VP of manufacturing at Amgen. You can reach him at witchermf@aol.com or on LinkedIn (linkedin.com/in/mark-witcher).
Mark F. Witcher, Ph.D., has over 35 years of experience in biopharmaceuticals. He currently consults with a few select companies. Previously, he worked for several engineering companies on feasibility and conceptual design studies for advanced biopharmaceutical manufacturing facilities. Witcher was an independent consultant in the biopharmaceutical industry for 15 years on operational issues related to: product and process development, strategic business development, clinical and commercial manufacturing, tech transfer, and facility design. He also taught courses on process validation for ISPE. He was previously the SVP of manufacturing operations for Covance Biotechnology Services, where he was responsible for the design, construction, start-up, and operation of their $50-million contract manufacturing facility. Prior to joining Covance, Witcher was VP of manufacturing at Amgen. You can reach him at witchermf@aol.com or on LinkedIn (linkedin.com/in/mark-witcher).